[What can be said about q and k immediately after an increase in Temperature] – So many ways to explore the “What can be said about q and k immediately after an increase in Temperature?”. Here so many websites are giving their answers and solutions. In this article, we explain what can be said about q and k immediately after an increase in Temperature. That means we will solve the problem and define the Temperature.
We want to give our best to result to readers to better understanding. So, I want to start with my article with good energy and confidence. Let’s start!There are different types of websites talking about this problem “What can be said about q and k immediately after an increase in temperature?”. That is very nice.
But some websites are not giving correct information about this question. Like “What can be said about q and k immediately after an increase in temperature?” So here I search so many sites of What can be said about q and k immediately after an increase in Temperature?.’ However , we will discuss “What can be said about q and k immediately after an increase in temperature?”. First of all, I will give you a definition of Temperature.
Table of Contents
What is Temperature?
The measure of coldness or hotness expression is called Temperature. As a student, we must know the exact meaning of Temperature. The Temperature in terms of some scales. That is Fahrenheit and Celsius. Temperature shows how heat energy will flow. For example, If a person’s body is hot, that is measured as a high temperature. Or if a person’s body is cold, that is counted as a lower Temperature.
Consider this system at equilibrium. A(aq)<—>B(aq) deltaH=-750 kJ/mol
Consider this scheme at equilibrium. A(aq)<—>B(aq) deltaH=-750 kJ/mol What can be said about Q and K instantly after an increase in Temperature? A) Q > K because Q increased B) Q > K because K decreased C) Q < K because Q decreased D) Q < K because K increased E) Q = K because neither is charged. How will the system reply to a temperature increase? A) shift left B) shift right C) no change
Concepts and Reason
The balance continuous (K) of the response gives the relation between the attention of the products. The response measure is the calculation of the random stage of the reaction.
Le chatelier’s principle explains The result of temperature revolution on equilibrium.

[What can be said about Q and K immediately after an increase in Temperature?]
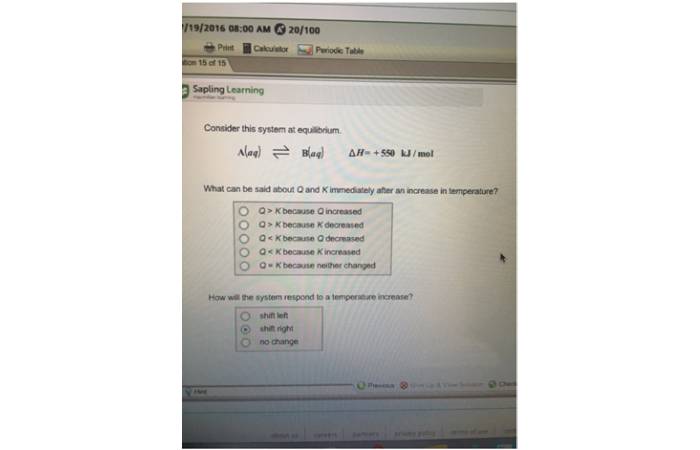
Consider the following equilibrium system.
A(aq)B(aq)H = +550 kJ/mol
[What can be said about Q and K immediately after a temperature increase?]
So, I want to double-check my understanding and reasoning for the solution.
Enthalpy has increased, indicating an endothermic reaction in which heat was introduced into the system to produce products. Hence, as heat increases, the system moves to the right, and more products are made. The equilibrium constant, K, will vary when the temperature changes. In this case, due to the increased heat, there will be more products. As a result, K will also increase, meaning that K > Q.
A positive enthalpy indicates that the reaction is endothermic. And heat is a reactant that must be added for the forward reaction to occur. When the Tem-perature rises, K rises, but Q does not change because the concentrations remain constant. As a result, Q K and the reaction tend towards products.




